SPAIN – INBRAIN Neuroelectronics, a pioneering company in brain-computer interface(BCI) therapeutics (BCI-Tx), has successfully closed a US $50 million Series B funding round.
The financing was spearheaded by imec.xpand, with additional participation from notable new investors including the EIC Fund, Fond ICO Next Tech, CDTI-Innvierte, and Avançsa.
Returning investors such as Asabys Partners, Aliath Bioventures, and Vsquared also contributed, marking a significant milestone that brings INBRAIN’s total funding to US $68 million since its inception.
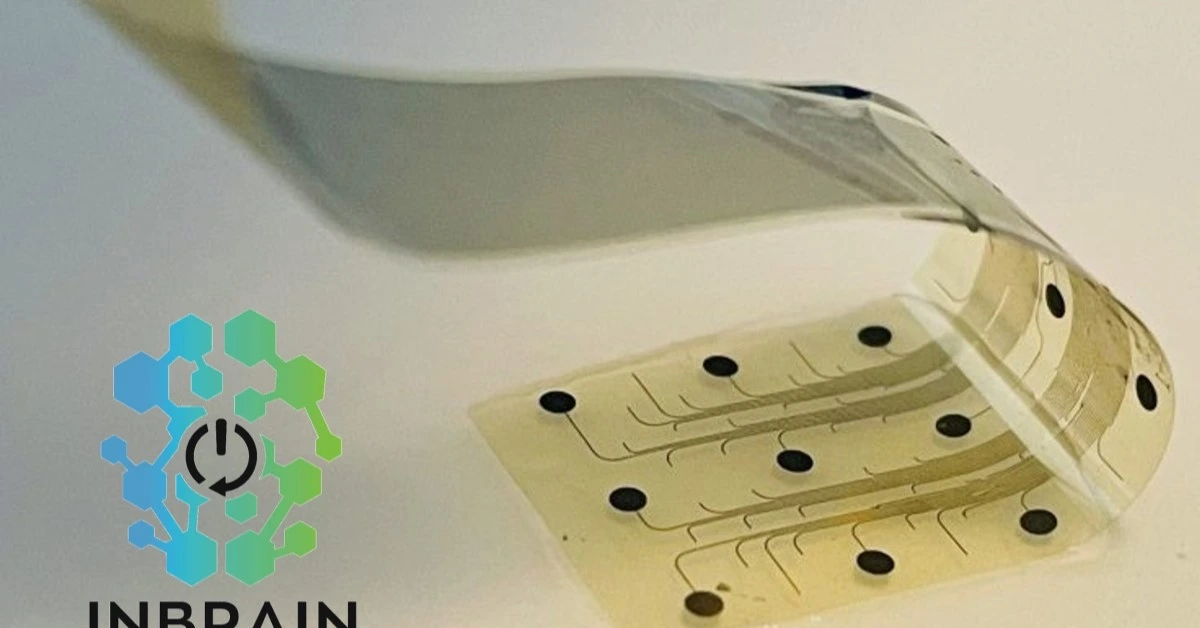
This influx of capital is set to bolster INBRAIN’s groundbreaking work in neural technology, leveraging the unique attributes of graphene—a Nobel Prize-winning material lauded for its unmatched conductivity, flexibility, and strength.
This innovative approach positions INBRAIN at the forefront of precision neurology, with its ultra-thin, 10-micrometer implant that can decode and modulate neural signals with exceptional accuracy.
Clinical expansion supported by Merck partnership
Further strengthening its clinical advancements, INBRAIN has also secured strategic support and funding from Merck KGaA.
This collaboration aims to advance the clinical development of INBRAIN’s BCI technology in areas aligned with Merck’s therapeutic focus.
The alliance underscores the significant potential for INBRAIN’s platform to impact both central and peripheral nervous system applications.
“Our partnership with Merck is a testament to the promise of our graphene-based platform,” stated an INBRAIN representative.
“This collaboration will fast-track our journey toward meaningful clinical applications, expanding the possibilities for patient care.”
Graphene: A New Era for Brain-Computer Interfaces
Graphene’s exceptional qualities set it apart from other materials used in neural interfaces.
As the thinnest and strongest known material, it provides superior electrical conductivity and flexibility, making it ideal for delicate neural applications.
INBRAIN’s implant, thinner than a human hair, has already shown promise in safely decoding and modulating brain activity.
The funding will enable INBRAIN to continue its clinical trials and further develop its AI-powered platform for treating neurological diseases.
This initiative aligns with the company’s mission to harness the precision of graphene technology to elevate the standards of neurology and neural treatment.
Collaborative efforts with Imec for commercial scaling
As part of the funding, INBRAIN announced a new collaboration with imec, the world’s leading independent nanoelectronics hub.
This partnership will facilitate the preparation for commercial-scale production of graphene interfaces, ensuring that the technology can be manufactured and deployed efficiently.
First Human Trials: A Promising Start
INBRAIN recently marked a pivotal achievement by successfully applying its graphene-based BCI in a human clinical trial at Salford Royal Hospital in Manchester, UK.
This ongoing trial is focused on assessing the safety of the device in brain cancer patients, with plans to include up to ten participants.
The study aims to demonstrate graphene’s superiority over conventional materials used in brain-computer interfaces.
The wider field: Synchron’s less invasive approach
While INBRAIN pushes forward with cutting-edge graphene technology, other players are also making strides in the BCI field.
One notable contender is Synchron, a U.S.-based company backed by high-profile investors Jeff Bezos and Bill Gates.
Synchron’s technology, known as the Stentrode, stands out for its minimally invasive implantation process.
Unlike Elon Musk’s Neuralink, which involves drilling into the skull, the Stentrode is inserted via the jugular vein, reducing surgical risks.
Tom Oxley, Synchron’s founder, emphasized the importance of non-invasive solutions for patients with severe paralysis. “Our aim has always been to make the technology as patient-friendly as possible,” said Oxley.
Synchron’s device, implanted into the brain’s blood vessels, can detect brain signals and transmit them to a chest-implanted receiver that communicates wirelessly with external devices.
This system has proven effective for patients with conditions like amyotrophic lateral sclerosis (ALS) and severe spinal injuries.
Despite challenges, including financial struggles in 2020, Synchron made a significant breakthrough with a US $40 million investment from Khosla Ventures and raised US $75 million in 2022.
The company has completed its first major FDA clinical trial, confirming the safety of its device, further cementing its position as a formidable player in the field.
Competitive landscape: Pioneering the future of BCI
The BCI sector is witnessing intense competition as various companies aim to refine their approaches to neural interfaces.
High-profile firms like Neuralink and Blackrock Neurotech often capture public attention with their ambitious projects and substantial funding.
However, lesser-known innovators, such as INBRAIN and Synchron, contribute significantly to the dynamic landscape with their unique, patient-centric technologies.
Oxley acknowledged Musk’s impact on the industry, mentioning their conversation during Synchron’s initial U.S. trials.
“It’s clear that innovation in this field benefits from a variety of approaches, and while our strategies differ, the goal is the same: improving patient outcomes,” he noted.
As the race to enhance neural interface technology continues, the field remains a hotbed for research and development.
The focus on reducing invasiveness, ensuring biocompatibility, and expediting regulatory approval is shaping the future of BCIs and their integration into modern medicine.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved