NETHERLANDS – Dutch medtech developer Philips has initiated a clinical trial in the U.S. to evaluate a pioneering treatment for peripheral artery disease (PAD), a condition characterized by narrowed arteries that restrict blood flow to the arms and legs.
PAD occurs when fatty deposits build up in the arteries, leading to atherosclerosis and reduced blood flow, which can result in pain, ulcers, and even amputations.
“We believe this combined approach has the potential to simplify the workflow, reduce risks, and improve patient outcomes,” Philips said.
Traditional treatments for PAD often involve multiple procedures and devices, increasing complexity and risk.
Other treatments for peripheral artery disease (PAD) include peripheral artery bypass surgery, which aims to reroute blood flow around blocked arteries, as well as antiplatelet medications like aspirin or clopidogrel, which help prevent blood clots.
Additionally, leg pain medications such as cilostazol may be prescribed to improve walking distance and reduce discomfort associated with PAD.
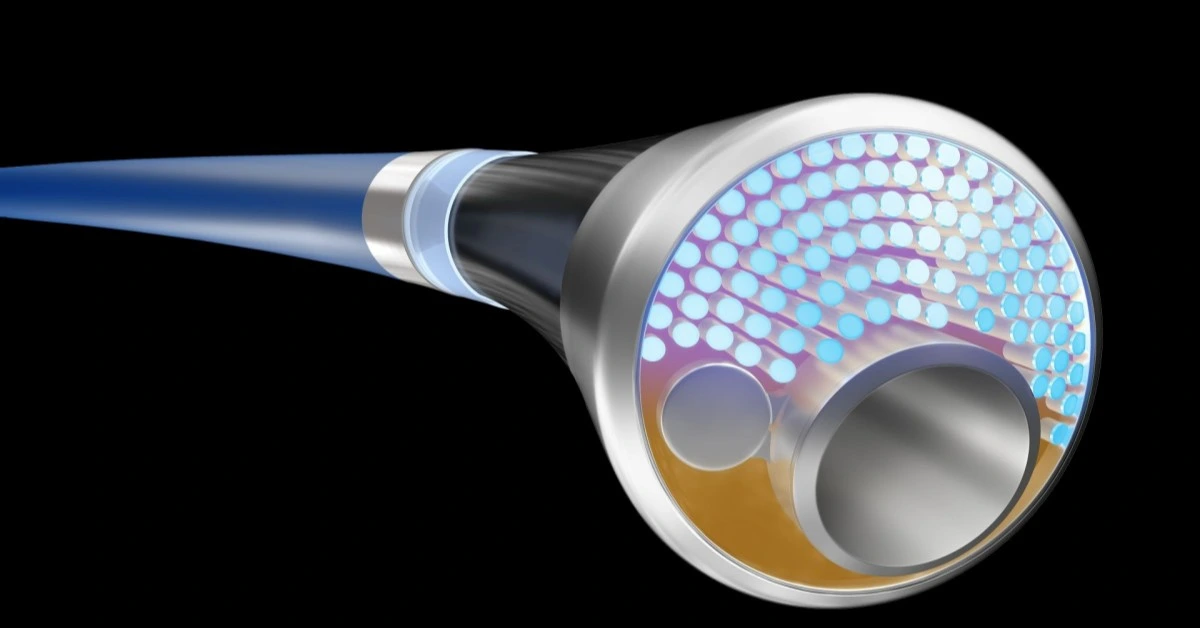
Unlike conventional intravascular lithotripsy systems, which rely on separate ultrasound catheters to create sonic shockwaves, Philips’ device uses a pulsed laser to create bubbles that generate the necessary shockwaves, offering a more integrated and efficient solution.
First successful procedure marks trial launch
The first successful use of the device took place at the Cardiovascular Institute of the South in Louisiana, where a 78-year-old male patient with peripheral vascular disease was treated.
Now, the clinical trial will expand, enrolling up to 155 patients at 30 sites across the U.S. The goal is to assess how this innovative combination of therapies can optimize procedural efficiency and enhance patient outcomes.
“This innovative approach to vessel preparation could improve patient outcomes while minimizing the need for multiple therapies and interventions,” commented Dr. Elizabeth Genovese, co-principal investigator of the trial at the Hospital of the University of Pennsylvania.
“Integrating both atherectomy and intravascular lithotripsy into a single device has the potential to revolutionize the treatment of complex PAD cases, particularly those with severe calcifications.”
A new era for PAD treatment
Philips’ new laser catheter combines two essential treatments for PAD: atherectomy, which uses UV light to ablate plaque, and intravascular lithotripsy, which breaks up calcified deposits with sonic shockwaves.
The company believes this dual approach will simplify the treatment process, reducing the need for separate devices and minimizing complications.
Philips’ Stacy Beske, business leader for Philips Image Guided Therapy Devices, emphasized the significance of the innovation, stating, “Our combined laser atherectomy and intravascular lithotripsy device reflects our commitment to providing physicians with tools to tackle complex vascular challenges more efficiently and effectively.”
THOR IDE study to validate safety and efficacy
The trial, known as the THOR IDE study, is designed to assess the safety and efficacy of the device in a multicenter, single-arm study.
The trial will test a novel device that integrates two advanced laser-based therapies—atherectomy and intravascular lithotripsy—into a single platform aimed at improving PAD treatment outcomes.
The primary endpoints include freedom from major adverse events such as mortality, unplanned amputations, or clinically driven target lesion revascularization within 30 days, as well as achieving less than 50% residual stenosis post-procedure. The study will follow patients for 12 months.
“We are excited to see the first patient enrolled in this important clinical trial,” said Dr. Genovese. “This approach to treating complex femoropopliteal lesions could revolutionize the care of PAD patients, especially those with moderate to severe calcifications.”
Currently investigational, the Philips laser-based PAD treatment system is not yet commercially available but shows great promise in transforming PAD care.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved