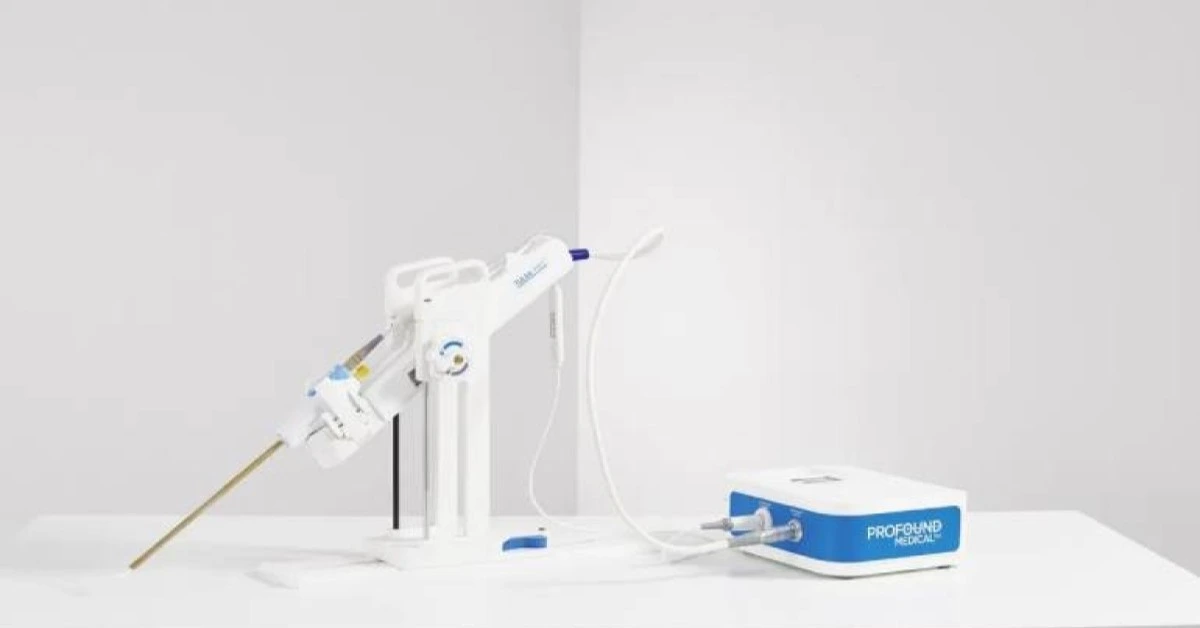
USA – Profound Medical has entered into a co-sales and co-marketing agreement with Siemens Healthineers to commercialize a cutting-edge MRI-guided prostate therapy solution.
This innovative partnership combines Profound’s TULSA-PRO system with Siemens Healthineers’ Magnetom Free.Max MR scanner, aiming to revolutionize prostate disease treatment.
The new agreement builds on a non-exclusive collaboration announced earlier in 2024, which also focused on integrating the TULSA-PRO and Magnetom Free.Max technologies.
The TULSA-PRO system is designed to perform transurethral ultrasound ablation (TULSA), an advanced, incision-free, and radiation-free procedure that uses real-time MRI guidance for precise treatment.
By gently heating prostate tissue through sound absorption technology, the system effectively ablates either whole- or partial-gland prostate tissue.
It is used for patients with low-, intermediate-, or high-risk prostate cancer and benign prostatic hyperplasia (BPH).
Notably, the TULSA procedure helps preserve urinary continence and sexual function while offering relief to patients undergoing active surveillance or experiencing BPH symptoms.
Complementing this technology, the Magnetom Free.Max MR scanner enhances clinical applications with the benefits of a mid-field MR scanner.
Its design improves access for larger or claustrophobic patients, expanding its utility to diverse healthcare settings, including orthopedic centers, rural hospitals, and ambulatory surgical centers (ASCs).
The companies plan to launch their integrated solution, branded as “TULSA+”, in 2025, pending compatibility testing between the TULSA-PRO system and Magnetom Free.Max scanner.
Leveraging AI to enhance prostate therapy
Earlier this year, Profound Medical achieved a significant milestone when the FDA cleared its AI-driven Contouring Assistant module for use with the TULSA-PRO system.
This machine learning-based tool efficiently delineates the prostate and targets ablation areas, utilizing a database of successful patient outcomes to ensure accurate treatment margins.
“The current clinical data shows that 20% of the time when you surgically remove the prostate, you are leaving some positive margins,” Profound’s CEO Arun Menawat shared in an interview.
He added that the module reduces surgery time by a third and expands the procedure’s accessibility to patients with comorbidities such as diabetes.
Additionally, the FDA had previously cleared Profound’s AI-assisted Thermal Boostmodule, which temporarily increases ablation target temperatures to treat advanced-stage prostate cancer.
Donald Hardie, Siemens Healthineers’ Head of Global Marketing & Sales Magnetic Resonance, expressed excitement about presenting their shared vision with Profound Medical for a comprehensive MRI-guided prostate therapy solution during the physician peer-to-peer “PRO-TALK Live!” event held in September 2024.
Similarly, Arun Menawat, the CEO and Chair of Profound Medical, highlighted that the co-sales and co-marketing agreement with Siemens Healthineers aligns seamlessly with their goal of broadening access to the TULSA technology.
He emphasized that with CMS now recognizing the TULSA procedure’s value at Urology APC Level 7, the collaboration offers a cost-effective, high-quality imaging solution suitable for ambulatory surgical centers (ASCs), hospitals, and even physicians’ offices.
Menawat also pointed out that the Magnetom Free.Max delivers imaging quality comparable to larger 1.5T scanners, making it ideal for facilities already requiring advanced MR imaging for both orthopedic and urological treatments.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved