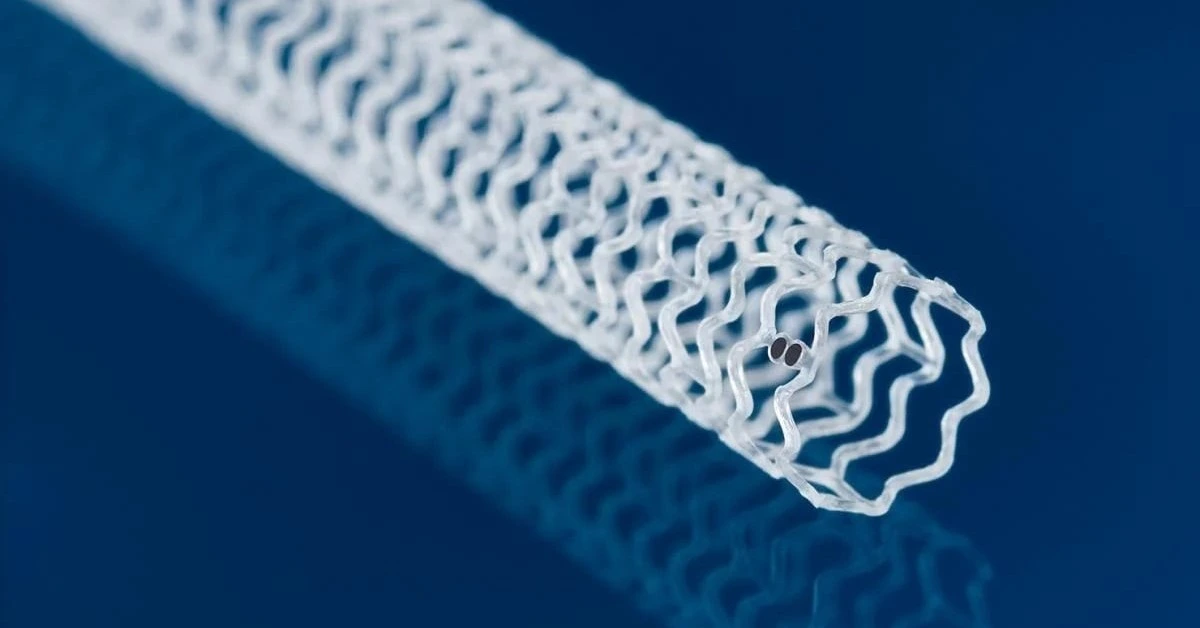
USA – Abbott has reported encouraging, sustained outcomes for its Esprit BTK drug-eluting stent in managing peripheral artery disease (PAD) below the knee, especially for patients with chronic limb-threatening ischemia (CLTI).
Approved by the FDA in April, the Esprit BTK stent is designed to hold arteries open and release the drug everolimus to support healing before dissolving naturally, leaving the vessel strong enough to stay open on its own.
Jennifer Jones-McMeans, divisional vice president of global clinical affairs at Abbott’s vascular division, highlighted, “The positive results at two years reinforce Esprit BTK’s potential to revolutionize the treatment of peripheral artery disease below the knee.”
The Esprit BTK system, which uses a dissolvable material similar to surgical sutures, is implanted through a minimally invasive procedure that requires only a small leg incision.
Once in place, it gradually dissolves over three years, providing temporary support while the vessel heals.
Abbott’s LIFE-BTK trial, a global study presented at VIVA 2024 in Las Vegas, assessed the device’s efficacy in over 260 patients with severe PAD.
The trial compared Esprit BTK to balloon angioplasty, the current standard treatment for PAD.
After two years, Esprit BTK outperformed balloon angioplasty in multiple areas. “We’re proud to be at the forefront of developing innovative treatment options for the millions of people living with PAD,” said Jones-McMeans.
The data showed that 90.3% of patients with Esprit BTK avoided the need for additional intervention, compared to 67.2% in the angioplasty group, representing a significant reduction in repeat procedures.
Higher freedom from severe symptoms and fewer re-narrowing events
Esprit BTK demonstrated higher freedom from CLTI symptoms, a severe manifestation of PAD, with a rate of 61.5% compared to 32.8% in the balloon angioplasty group.
Additionally, the one-year data indicated a 35.2% improvement in reducing vessel re-narrowing in the Esprit group, supporting its long-term effectiveness in keeping arteries open.
In addition to the LIFE-BTK trial, Abbott has launched a post-approval study to assess Esprit BTK’s safety and effectiveness in everyday clinical settings.
Dr. Bernardino L. Rocha, a vascular surgeon in Oklahoma City, enrolled the first patient in this new study.
Abbott aims to confirm that the Esprit BTK stent offers a reliable solution for PAD patients, reducing the need for multiple interventions and, in some cases, helping patients avoid amputation.
“This innovation is ultimately helping people live fuller, better lives by eliminating the need for multiple interventions,” Jones-McMeans added.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved