USA – BD (Becton, Dickinson and Company), a global leader in medical technology, has launched a new U.S.-based patient data registry to assess the real-world performance of its Rotarex Atherectomy System.
The registry, named XTRACT, will track clinical outcomes in patients with peripheral artery disease (PAD) who are treated with the system.
This prospective, post-market study will enroll up to 600 patients across 100 sites in the United States.
Led by Co-Principal Investigators Dr. Prakash Krishnan, an interventional cardiologist, and Dr. Todd Berland, a vascular surgeon, the study will monitor patients at 30 days, 6 months, and 12 months after treatment to measure safety and effectiveness.
“The Rotarex System has shown strong results in international studies. Now, through the XTRACT Registry, we aim to understand how it performs in real-world cases across the U.S.,” said Dr. Krishnan. “This data will help clinicians make better decisions and improve treatment outcomes.”
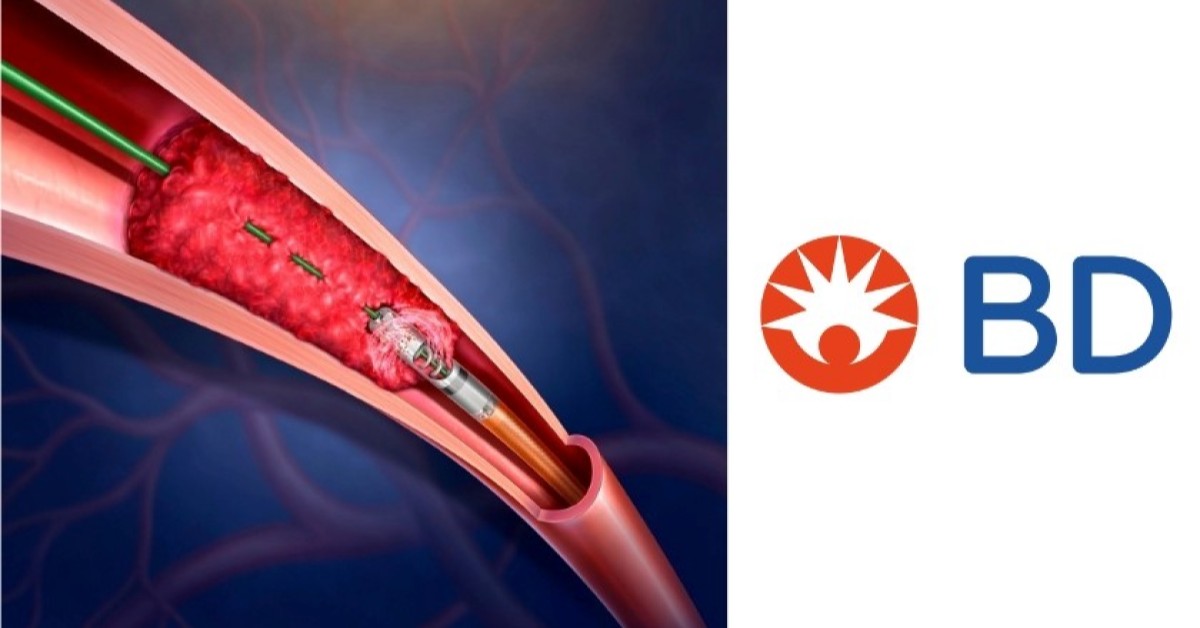
The Rotarex Atherectomy System is a minimally invasive device that removes both plaque and blood clots from peripheral arteries.
With dual capabilities for atherectomy and thrombectomy, it provides physicians with a versatile tool to manage complex peripheral artery disease (PAD) lesions.
“This is the first large-scale U.S. registry focused on the Rotarex System,” said Rima Alameddine, worldwide president of BD Interventional-Peripheral Intervention.
“We are committed to helping physicians deliver better care by providing evidence-based insights from real patients.”
Peripheral artery disease affects over 21 million Americans and 200 million people worldwide. Left untreated, it can lead to serious complications, including heart disease and limb amputation.
Through the XTRACT Registry, BD aims to strengthen clinical understanding, support innovation, and ultimately improve quality of life for patients living with PAD.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved