SWITZERLAND – Roche has secured FDA approval for its eye implant, Susvimo, as a treatment for diabetic retinopathy, expanding its use to a third major eye condition.
This decision follows encouraging results from the Phase III PAVILION trial, where the implant showed strong benefits over standard care.
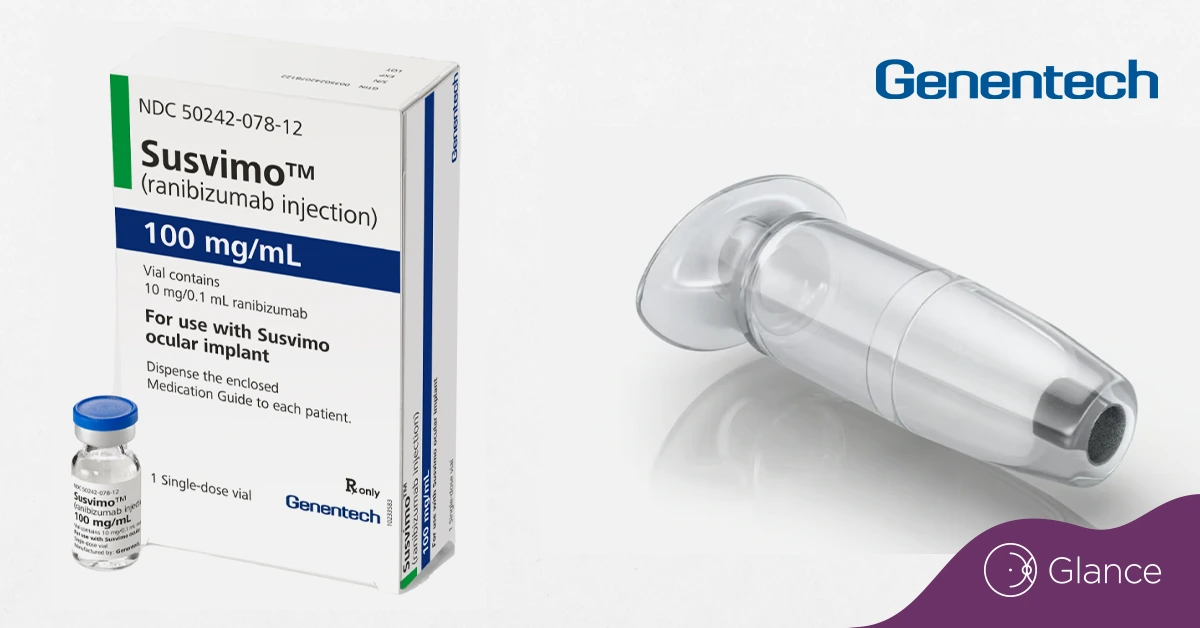
Susvimo is a refillable device that delivers a steady dose of ranibizumab, a VEGF inhibitor, and is designed to be refilled just once every nine months.
In the trial, patients using the implant were significantly more likely to show a two-step improvement on the Diabetic Retinopathy Severity Scale (DRSS), a measure linked to reduced risk of severe vision loss.
Notably, none of the patients using Susvimo required extra treatment at the one-year point.
Originally approved in 2021 for wet age-related macular degeneration, Susvimo was later pulled from the market due to performance issues.
Roche made improvements to the implant and refill system and relaunched it in the U.S. in mid-2023. It has since been cleared for diabetic macular oedema (DME) as well.
Despite the regulatory green light, Roche may face a challenge in gaining physician trust.
According to Genentech’s press release, the medication is now available to US retina specialists for use in patients with DR who have responded to at least two anti-vascular endothelial growth factor (anti-VEGF) injections.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved