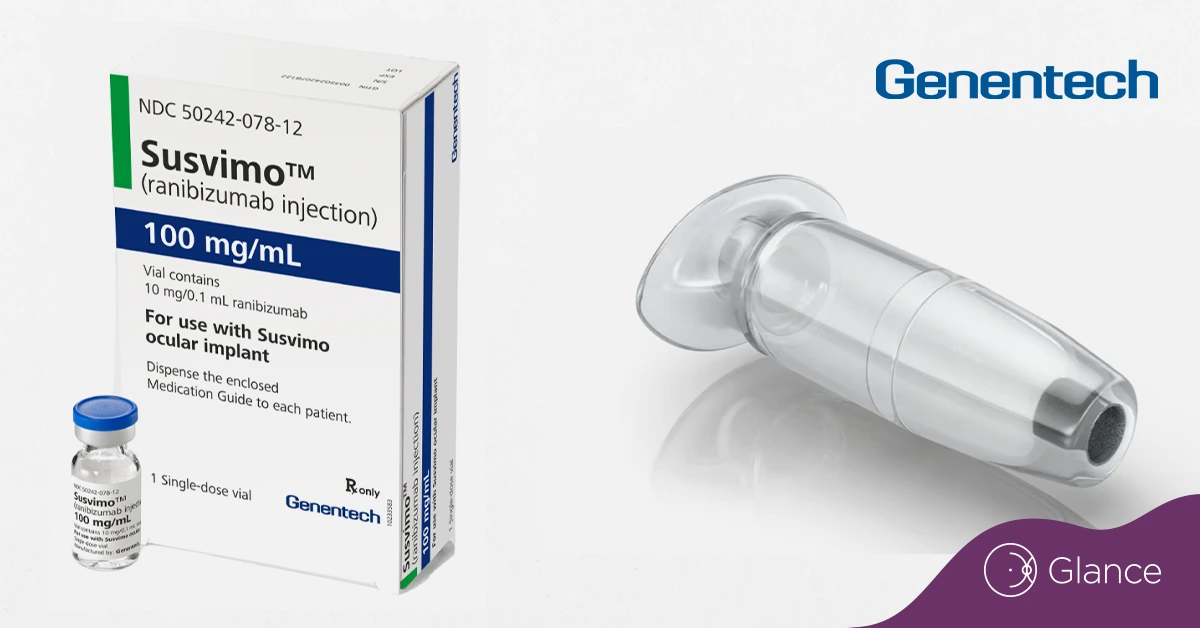
SWITZERLAND – Roche’s Susvimo implant, a refillable device for treating eye diseases, has received expanded approval from the U.S. FDA.
It is now authorized for use in patients with diabetic macular edema (DME), a major cause of vision loss. This new approval follows Susvimo’s 2021 approval for the treatment of wet age-related macular degeneration (AMD), adding to the growing potential of this innovative device.
Susvimo was temporarily withdrawn from the market in 2022 due to a manufacturing issue involving a defective seal in the implant.
This defect caused the active drug to leak out too quickly. After addressing the issue, Roche reintroduced the device last year.
The implant offers a much-needed alternative to frequent eye injections, with some treatments requiring up to monthly injections.
The new FDA approval now positions Susvimo as “the first and only continuous delivery treatment” for DME, a condition affecting more than 29 million people worldwide.
The implant uses a portal delivery system (PDS) the size of a grain of rice and continuously delivers medication for at least six months.
This allows patients to manage DME with just two treatments per year, compared to the frequent injections traditionally required.
The approval for DME could significantly boost Roche’s sales expectations for Susvimo, as the DME patient population is larger and growing in line with increasing diabetes rates globally.
The active ingredient in Susvimo is ranibizumab, the same VEGF inhibitor found in Roche’s Lucentis, which has long been used to treat AMD, DME, and other eye diseases. However, Lucentis lost patent protection in 2021, leading to a sharp decline in sales.
Susvimo and another Roche product, Vabysmo (faricimab), were both intended to help maintain Roche’s eye disease treatment franchise.
While Susvimo faced setbacks, Vabysmo has performed well, generating over $4.3 billion in 2024 sales.
Although Roche has not yet provided detailed sales projections for Susvimo, analysts had originally estimated it could bring in US $1 billion or more annually.
With the new DME approval, Roche hopes to capture market share from competitors like Bayer and Regeneron, whose Eylea (aflibercept) has a new high-dose format approved by the FDA that reduces injection frequency.
Susvimo has also been praised for improving patient experience, as it reduces the need for frequent injections.
However, concerns over endophthalmitis (inflammation of the intraocular fluids) have been raised by ophthalmologists, which could limit widespread adoption.
Roche is continuing to explore new uses for the Susvimo implant, including its potential for delivering a new bispecific antibody treatment designed to work with the device.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved