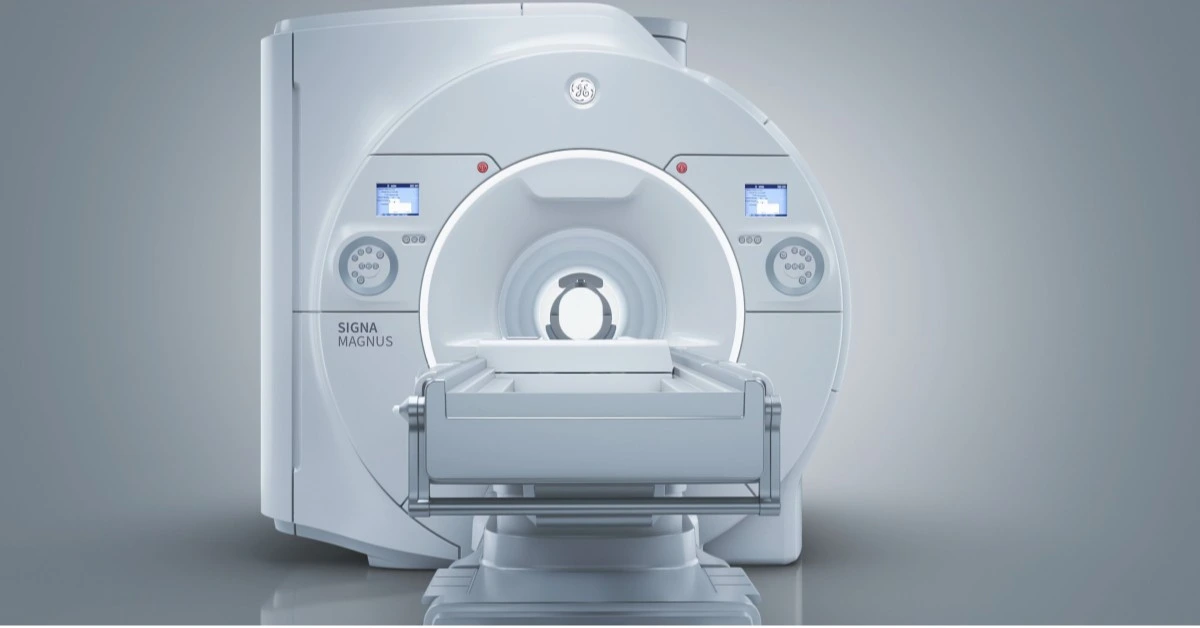
USA – GE Healthcare has announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its latest innovation, the Signa Magnus—a head-only MRI system designed to redefine neuroimaging.
The system features a cutting-edge asymmetrical gradient coil specifically tailored for high-resolution brain scans, enabling enhanced imaging precision while addressing patient comfort.
Jason Polzin, GE Healthcare’s General Manager for MR Applications Platform and Research Technologies, emphasized the significance of this milestone:
“Obtaining FDA clearance further validates our commitment to not only innovating but also delivering clinical technologies that have real-world impact.”
Unlike traditional whole-body MRI scanners, Signa Magnus is engineered with a reduced inner diameter coil, allowing it to achieve higher gradient amplitude and slew rates—critical factors for detailed imaging.
Its unique asymmetrical design shifts the gradient isocenter closer to the patient’s head, improving access and minimizing constraints caused by shoulder width.
“This head-only design enables imaging capabilities far beyond conventional systems, making it a significant advancement in MRI technology for neuroimaging applications,” GE Healthcare stated.
Key benefits of the Signa Magnus include:
Transforming neurological care
The system builds on GE’s established Signa Premier MRI platform, with significant enhancements to magnetic field gradients.
It offers precision imaging for conditions such as neurological diseases, psychiatric disorders, and cancers, making it an invaluable tool for early diagnosis and treatment planning.
Stanford University professor Kawin Setsompop praised its potential: “I plan to leverage the gradient performance to look at microstructures with diffusion imaging, such as axonal diameter. The system’s features will help achieve higher resolution with fewer artifacts.”
Easy integration for medical facilities
GE Healthcare plans to offer the Signa Magnus both as a standalone system and as an upgrade for existing Signa Premier machines, enabling facilities to adopt this cutting-edge technology without extensive infrastructure changes.
Investigational installations of the Signa Magnus are already underway at prestigious institutions such as Walter Reed National Military Medical Center and the University of Wisconsin-Madison.
These systems are being used to explore new biomarkers for neurodegenerative disorders and advance research in brain imaging.
XRP HEALTHCARE L.L.C | License Number: 2312867.01 | Dubai | © Copyright 2025 | All Rights Reserved